Mytesi Sales Increased 17.71% Each Month Over the Prior Month
SAN FRANCISCO--(BUSINESS WIRE)--Jan. 22, 2018--
Jaguar Health, Inc. (NASDAQ: JAGX) (Jaguar or the Company), a commercial
stage natural-products pharmaceuticals company focused on developing
novel, sustainably derived gastrointestinal products for both human
prescription use and animals on a global basis, today provided the
following updates regarding commercial, educational and product
development programs and 2017 results.
This press release features multimedia. View the full release here:
http://www.businesswire.com/news/home/20180122006522/en/
(Graphic: Business Wire)
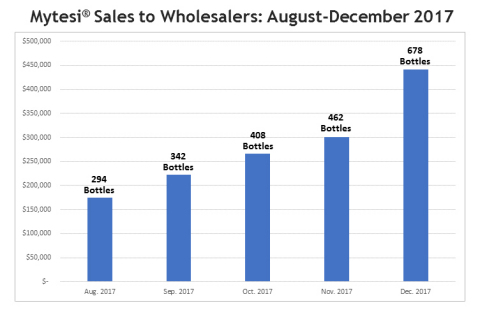
Mytesi® Sales Update (August 2017 – December
of 2017)
Effective July 31, 2017, Jaguar merged with Napo Pharmaceuticals, Inc.
(Napo) and acquired its first-in-class, FDA-approved anti-secretory
human prescription drug product, Mytesi® (crofelemer). For
the period August 1, 2017 through December 31, 2017, preliminary sales
of Mytesi® were approximately $1.40 million. During the
aforementioned period, Mytesi® sales increased more than 78%
relative to average monthly Mytesi® sales that took place
from January through July of 2017, which is before marketing efforts
were deployed in the combined Company and the deployment in the fourth
quarter of Napo’s team of nine dedicated sales reps. On average during
the August 1, 2017 through December 31, 2017 period, sales increased
17.71% each month over the prior month.
Six of Napo’s nine highly trained Mytesi® sales
representatives are former long-term employees of the HIV portfolio
business of drugmaker Bristol-Myers Squibb, while the remainder of the
team possess extensive experience in drug sales to both HIV healthcare
providers and gastroenterologists.
“Napo has identified a total high-potential prescriber base of 3,500
high-volume ART prescribers (HIV specialists) and 1,500
gastroenterologists who see the highest number of people living with
HIV. The Napo sales force is located in the U.S. geographies with the
highest potential, and is targeting the prescribers with the highest
potential to drive Mytesi® business. From September 1, 2017
through November 30, 2017, there was an increase of 86% in new Mytesi®
prescribers among gastroenterologists and 8% in HIV specialists,
coincident with the deployment of our direct sales force,” Lisa Conte,
Jaguar's President and CEO, stated. “According to data provided by
IQVIA, the number of Mytesi® prescriptions written by
physicians increased an average of 9.5% each month over the prior month
during the August 1, 2017 through December 31, 2017 period.
Additionally, patient redemptions of our Mytesi® Copay
Savings Card increased an average of 7% each month over the prior month
during the same period.”
Sales is a measure that is recognized under accounting principles
generally accepted in the United States of America (“GAAP”), in
accordance with new 2018 revenue recognition rules, still under review
by the Company and its auditors. As a result, the preliminary Mytesi®
sales figures provided herein are subject to revision until the Company
reports final fourth quarter fiscal 2017 results no later than March 30,
2018.
Commercialized directly by Napo in the U.S., Mytesi® is the
only antidiarrheal studied in and U.S. FDA-approved for the symptomatic
relief of noninfectious diarrhea in adults living with HIV/AIDS on
antiretroviral therapy (ART). Mytesi® is a prescription
treatment for diarrhea that works differently, by acting locally in the
GI tract to normalize the flow of water. Mytesi® does not
have drug-drug interactions with ART and does not affect GI motility.
Napo Launches Health Care Practitioner and Patient Advocate Speaker
Bureau – Plans to Conduct More Than 1,400 Educational Events in 2018
Napo recently completed the training of 29 health care practitioners
(HCPs) and ten patient advocates to serve as members of the Napo
Speakers Bureau. Medical education presentations led by participating
HCPs—a group that includes HIV/AIDS specialists, infectious disease
specialists, gastroenterologists, colorectal surgeons, and nurse
practitioners—will focus on the prevalence and pathophysiology of
gastrointestinal consequences of HIV infection and on the latest
treatment options for HIV-related diarrhea. Presentations given by
patient advocate members will provide information to people living with
HIV (PLWH) about the prevalence of diarrhea in PLWH and offer guidance
about talking to HCPs regarding diarrhea-related concerns.
As part of Napo’s medical and patient education program, the Mytesi®
direct sales force are planning more than 1,400 live and virtual
educational events for 2018. Live events will largely take place in the
following key geographies covered by the Mytesi® sales team:
Miami/South Florida, Los Angeles/Palm Springs, New York, Houston,
Chicago/St. Louis, Indianapolis, Kansas City, Alabama, Atlanta, San
Francisco, DC, Pennsylvania, New Jersey, Delaware, Maryland, Mississippi
and Louisiana.
“Since the merger of Jaguar and Napo, we have both initiated and
dramatically expanded Mytesi®-related commercial, educational
and development initiatives, including the creation of our direct sales
force and our scientific advisory board, the roll-out of
direct-to-consumer advertising campaigns, publication-focused efforts,
government affairs activities regarding neglected comorbidities of HIV,
and the launch of the Napo Speaker Bureau,” Conte commented. “Mytesi®
sales and the awareness of a novel first-in-class approach to managing
diarrhea tested specifically in the HIV population are clearly
benefiting from the programs we’ve put in place—in addition to the
efforts of our recently expanded team of experienced HIV sales
representatives.”
Napo Accepts Request to Support Investigator-Initiated Trial of
Crofelemer
Napo has accepted a request for support submitted by Dr. Mohamad
Miqdady, Chief of Pediatric Gastroenterology, Hepatology and Nutrition
at Sheikh Khalifa Medical City (SKMC) in Abu Dhabi, for an
investigator-initiated trial of crofelemer, the active pharmaceutical
ingredient in Mytesi®, for congenital diarrheal disorders
(CDDs) in children.
CDDs are a group of rare, chronic intestinal channel diseases, occurring
exclusively in early infancy, that are characterized by severe, lifelong
diarrhea and a lifelong need for nutritional intake either parenterally
or with a feeding tube. CDDs are related to specific genetic defects
inherited as autosomal recessive traits. The incidence of CDDs is
prevalent in regions where consanguineous marriages (related by blood)
is part of the culture. CDDs are directly associated with serious
secondary conditions including dehydration, metabolic acidosis, and
failure to thrive, prompting the need for immediate therapy to prevent
death and limit lifelong disability.
SKMC is the Abu Dhabi public health system’s flagship institution and
the largest hospital in the United Arab Emirates (UAE), consisting of a
586-bed tertiary hospital, 14 outpatient specialty clinics, and the Abu
Dhabi Blood Bank, all of which are accredited by Joint Commission
International, the oldest and largest healthcare standards-setting and
accrediting body in the United States. Dr. Miqdady is American Board
certified in Pediatric Gastroenterology, Hepatology and Nutrition, and
he is a member of Napo’s Scientific Advisory Board.
Napo intends to submit documentation in the first half of 2018 to the
US. Food and Drug Administration (FDA) for the planned formulation ofcrofelemer appropriate for feeding tube administration to support
this investigation.
As announced on June 5, 2017, Napo has received orphan drug designation
from the FDA for short bowel syndrome (SBS). The Orphan Drug Act
provides for granting special status to a drug or biological product to
treat a rare disease or condition upon request of a sponsor. Orphan
designation qualifies the sponsor of the drug for various development
incentives, including extended exclusivity, tax credits for qualified
clinical testing, and relief of filing fees.
Investigator-Initiated Trials of Crofelemer in Cancer Therapy-related
Diarrhea (CTD)
As previously announced, an investigator-initiated trial titled HALT-D:
DiarrHeA Prevention and ProphyLaxis with Crofelemer in HER2 Positive
Breast Cancer Patients Receiving Trastuzumab, Pertuzumab, and Docetaxel
or Paclitaxel with or without Carboplatin is currently underway in
conjunction with Georgetown University. The primary objective of the
study is to characterize the incidence and severity of diarrhea in
patients receiving investigational therapy in the setting of
prophylactic anti-diarrheal management.
As also previously announced, a second study, titled An Open-Label
Study to Characterize the Incidence and Severity of Diarrhea in Patients
with Early-Stage HER2+ Breast Cancer Treated with Neratinib and
Intensive Loperamide Prophylaxis, is currently underway in
conjunction with the University of California, San Francisco. The study
is designed to evaluate crofelemer as a salvage anti-diarrheal therapy
used with the investigational breast cancer agent neratinib. The primary
objective is to characterize the incidence and severity of diarrhea in
patients with early-stage breast cancer receiving adjuvant trastuzumab
and neratinib followed by one year of neratinib monotherapy in the
setting of prophylactic anti-diarrheal management.
“Diarrhea continues to be an area of concern for patients undergoing
cancer treatment. Novel targeted agents, such as epidermal growth factor
receptor antibodies and tyrosine kinase inhibitors (TKIs), may block
natural chloride secretion regulation pathways in the normal
gastrointestinal mucosa, thereby leading to secretory diarrhea,” Conte
commented. “We recognize the importance of future development activities
on supportive care for patients being treated with these cancer-related
therapies, analogous to the supportive care of managing diarrhea in
people living with HIV/AIDS.”
Animal Health Updates
While Jaguar’s commercial and development efforts have evolved to focus
primarily on Mytesi® and human pipeline indications since its
merger with Napo, the Company is continuing animal health initiatives
related to Canalevia™, its drug product candidate for
treatment of various types of diarrhea in dogs, and Equilevia™,
its non-prescription, personalized, premium product for total gut health
in equine athletes.
As previously announced, Jaguar has received MUMS (Minor Use and Minor
Species) designation status from the FDA for Canalevia™ for
the indication of chemotherapy-induced diarrhea in dogs. Jaguar has
completed clinical and manufacturing activity for Canalevia™
for this indication. MUMS designation is modeled on the orphan-drug
designation for human drug development and offers possible financial
incentives to encourage MUMS drug development, such as the availability
of grants to help with the cost of developing the MUMS drug.
As announced last month, Jaguar has entered into a collaboration
agreement with Seed Mena Businessmen Services LLC (SEED) for Equilevia™.
Based in Dubai in the UAE, SEED is affiliated with Seed Group, a
diversified group of companies under the umbrella of The Private Office
of His Royal Highness Sheikh Saeed Bin Ahmed Al Maktoum establishing
strategic partnerships with multinational companies from around the
globe in an aim to leverage Seed Group’s network to support potential
business expansion in the MENA (Middle East and North Africa) region.
The UAE has become a global leader in horse racing, equine endurance
competitions, and other equine athletic activities. Gut health is of
critical importance in competitive horses, as conditions such as ulcers
can meaningfully impair equine athlete performance, and colic can lead
to the death of an otherwise healthy horse in a matter of hours.
According to a third-party 2005 study, as many as 55% of performance
horses have both colonic and gastric ulcers, and 97% of performance
horses have either a gastric (87%) or a colonic (63%) ulcer.1
Net sales in 2017 for Jaguar’s non-prescription Neonorm™ Foal
and Neonorm™ Calf products totaled approximately $422,000.
Collaboration revenue totaled approximately $2.9M. Jaguar continues to
maintain a relationship with the Company’s dairy market distributor in
addition to selling Neonorm™ directly to end users though
neonorm.com.
About Mytesi®
Mytesi® (crofelemer) is an antidiarrheal indicated for the
symptomatic relief of noninfectious diarrhea in adult patients with
HIV/AIDS on antiretroviral therapy (ART). Mytesi® is not
indicated for the treatment of infectious diarrhea. Rule out infectious
etiologies of diarrhea before starting Mytesi®. If infectious
etiologies are not considered, there is a risk that patients with
infectious etiologies will not receive the appropriate therapy and their
disease may worsen. In clinical studies, the most common adverse
reactions occurring at a rate greater than placebo were upper
respiratory tract infection (5.7%), bronchitis (3.9%), cough (3.5%),
flatulence (3.1%), and increased bilirubin (3.1%).
More information and complete Prescribing Information are available at
Mytesi.com. Crofelemer, the active ingredient in Mytesi®, is
a botanical (plant-based) drug extracted and purified from the red bark
sap of the medicinal Croton lechleri tree in the Amazon
rainforest. Napo has established a sustainable harvesting program for
crofelemer to ensure a high degree of quality and ecological integrity.
About Jaguar Health, Inc.
Jaguar Health, Inc. is a commercial stage natural-products
pharmaceuticals company focused on developing novel, sustainably derived
gastrointestinal products for both human prescription use and animals on
a global basis. Our wholly-owned subsidiary, Napo Pharmaceuticals, Inc.,
focuses on developing and commercializing proprietary human
gastrointestinal pharmaceuticals for the global marketplace from plants
used traditionally in rainforest areas. Our Mytesi®
(crofelemer) product is approved by the U.S. FDA for the symptomatic
relief of noninfectious diarrhea in adults with HIV/AIDS on
antiretroviral therapy.
For more information about Jaguar, please visit jaguar.health.
For more information about Napo, visit napopharma.com.
Forward-Looking Statements
Certain statements in this press release constitute “forward-looking
statements.” These include statements regarding the Company’s plan to
conduct more than 1,400 educational events in 2018, the
investigator-initiated trial of crofelemer for CDDs, the Company’s
intention to submit documentation in the first half of 2018 to the FDA
for the planned formulation ofcrofelemer to support this
investigation, the Company’s belief that crofelemer may have
considerable potential to help manage the severe diarrhea and
dehydration symptomatic of CDDs, and the Company’s plans to focus
upcoming resources on the development of potentially important
supportive care for patients being treated with cancer-related
therapies. In some cases, you can identify forward-looking statements by
terms such as “may,” “will,” “should,” “expect,” “plan,” “aim,”
“anticipate,” “could,” “intend,” “target,” “project,” “contemplate,”
“believe,” “estimate,” “predict,” “potential” or “continue” or the
negative of these terms or other similar expressions. The
forward-looking statements in this release are only predictions. Jaguar
has based these forward-looking statements largely on its current
expectations and projections about future events. These forward-looking
statements speak only as of the date of this release and are subject to
a number of risks, uncertainties and assumptions, some of which cannot
be predicted or quantified and some of which are beyond Jaguar’s
control. Except as required by applicable law, Jaguar does not plan to
publicly update or revise any forward-looking statements contained
herein, whether as a result of any new information, future events,
changed circumstances or otherwise.
1Pellegrini FL. Results of a large-scale necroscopic study of
equine colonic ulcers. J Equine Vet Sci. 2005;25(3):113-117.
Jaguar-JAGX

View source version on businesswire.com: http://www.businesswire.com/news/home/20180122006522/en/
Source: Jaguar Health, Inc.
Jaguar Health, Inc.
Peter Hodge
phodge@jaguar.health



